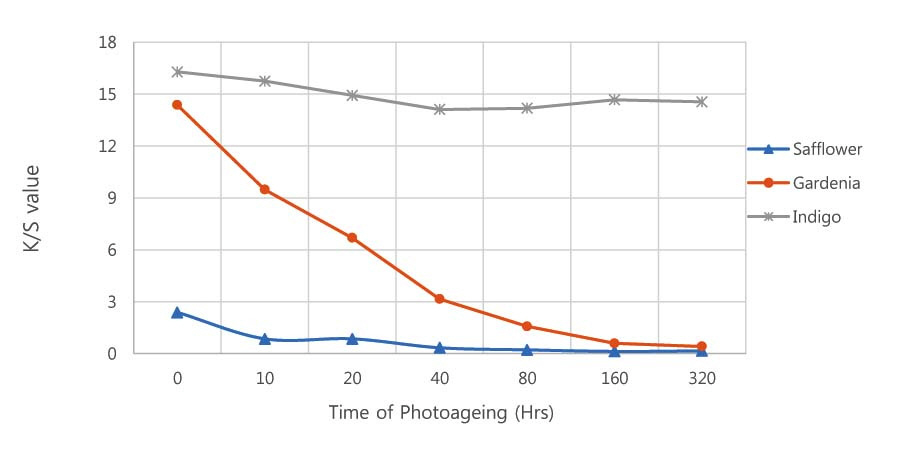
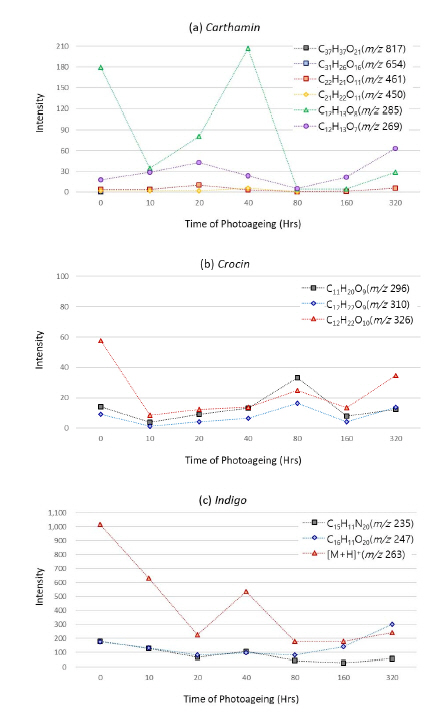
Aoyagi, S.,
2018,
Time-of-flight secondary ion mass spectrometry. Compendium of Surface and Interface Analysis., Singapore: Springer, 725-731.
Chen, K.,
Leona, M.,
Vo-Dinh, T.,
2007,
Surface enhanced Raman scattering for identification of organic pigments anddyes in works of art and cultural heritage material.
Sens. Rev., 27, (2), 109-120.

Chen, Y.,
Cai, L.,
Zhao, C.,
Xu, H.C.,
Cao, C.Y.,
Liu, Y.,
Jia, L.,
Yin, H.X.,
Chen, C.,
Zhang, H.,
2008,
Spectroscopic, stability and radical-scavenging properties of a novel pigment from gardenia.
Food Chem., 109, (2), 269-277.


FAO
1997, Carthamus yellow, in Compendium of Food Additive Specifications. Addendum 5. (FAO Food and
Nutrition Paper - 52 Add. 5), Rome: FAO, 198.
Kim, J.P.,
Lee, J.J.,
2003, Traditional dyes of Korea: Dyes and techniques. Seoul: Seoul National University Press, 87.
(in Korean)
Lee, J.H.,
Kang, M.H.,
Lee, K.B.,
Lee, Y.H.,
2013,
Characterization of natural dyes and traditional Korean silk fabric by surface analytical techniques.
Materials (Basel), 6,(5), 2007-2025.



Lee, Y.,
Lee, J.,
Kim, Y.,
Choi, S.,
Ham, S.W.,
Kim, K.J.,
2008,
Investigation of natural dyes and ancient textiles from Korea using TOF-SIMS.
Appl. Surf. Sci., 255, (4), 1033-1036.

Park, W.J.,
Kim, K.S.,
Kim, S.D.,
Park, J.H.,
Koh, J.S.,
2007,
Korean traditional red dyeing and characterization of its color properties by investigating old documents (I) - Dyeing red shades.
Journal of Textile Science and Engineering, 44, (4), 220-231.
Sato, K.,
Sugimoto, N.,
Ohta, M.,
Yamazaki, T.,
Maitani, T.,
Tanamoto, K.,
2003,
Structure determination of minor red pigment in carthamus red colorant isolated by preparative LC/MS.
Food Addit. Contam., 20, (11), 1015-1022.


Song, H.S.,
Kim, B.H.,
2004,
The beauty of natural dyed traditional colors., Seoul: Sookmyeong University Press, 208-209. (in Korean)