Composition of the Adhesive Used for Fixing Glass Eyes of the Stone Standing Maitreya of Daejosa Temple, Buyeo (Treasure No. 217)
Article information
Abstract
In the process of the conservation treatment of the glass eyes of the stone standing Maitreya of Daejosa temple, Buyeo (Treasure No. 217), a blackish material, expected to be the adhesive for fixing the glass eyes, was collected and analyzed. Infrared spectroscopy and pyrolysis/gas chromatography/mass spectrometry (pyrolysis/GC/MS) were employed to identify the organic material in the sample. The IR analysis revealed the presence of materials such as apatite or bone black. The pyrogram of the sample was similar to that of Asian lacquer, among traditional adhesives. In particular, the pyrolysis/GC/MS analysis with online methylation detected 1,2-dimethoxy-3-pentadecylbenzene, methyl 7-(2,3- dimethoxyphenyl) heptanoate, and methyl 8-(2,3-dimethoxyphenyl)octanoate. These are known to be the pyrolysis products of catechol and its oxidation product, which indicated the presence of Asian lacquer in the sample. X-ray diffraction, X-ray fluorescence, and thermal gravimetry analysis showed that the sample contained ca. 60% inorganic substances, including apatite. Radiocarbon dating of the sample suggested that the blackish material was applied between the late 13th and early 15th century, revealing some discrepancy with the art–historical manufacturing time of the Maitreya. From the above analysis, it was concluded that Asian lacquer and bone ash were used to attach the glass eyes by forming a thick blackish lacquer layer.
1. INTRODUCTION
Requests were received for the conservation treatment of the eyes of the stone standing Maitreya (Treasure No. 217) of Daejosa temple, Buyeo, Chungcheongnam-do in the course of its emergency maintenance. In the process of removing the glass eyes for the conservation treatment, the components of materials assumed to be the adhesive covering around the glass eyes were analyzed and the period in which they were used was estimated.
1.1. The eyes of stone standing Maitreya of Daejosa temple (Treasure No. 217)
The stone standing Maitreya of Daejosa temple, Buyeo (Treasure No. 217) is located in Imcheon-myeon, Buyeo, Chungcheongnam-do, South Korea. It is composed of massive coarse-grained biotite granite with a height of approximately 8.64 m and a maximum width of 4.2 m. It is unclear when the stone Maitreya was created; however, because its size and style are similar to those of stone standing Maitreya of Gwanchok temple, Nonsan, it is estimated that the date of creation was around 950–970 AD(Buyeogun, 1999;Jeong, 2007). The glass eyes were inserted by chiseling holes on the iris of the stone Maitreya which are slightly larger than an eye. It was assumed that after applying the adhesive inside the eye holes, the glass eyes were pushed in; then, while a part of the glass eye was still exposed, the adhesive was filled in between the glass eye and the hole, thereby bonding them(Figure 1).

Photographs and diagram of the object. (A) Stone standing Maitreya of Daejosa temple, Buyeo, (B) Glass eye and (C) Schematic representation of the installed glass eye.
When separating the glass eyes from the eye holes, after removing the outside adhesive, the glass eyes were detached and the adhesive remaining inside the holes was removed. Accordingly, the adhesives were collected and classified as either outside adhesive or inside adhesive. The adhesives were generally black and hard but felt light despite its dense structure. The amount of outside adhesive collected was smaller than that of the inside adhesive. Furthermore, when the adhesives were collected on site, the outside adhesive appeared to be brighter in color and lighter in weight than the inside adhesive, thereby showing some differences in properties(Figure 2).
1.2. Analysis of Asian lacquer-based traditional adhesive
Traditional adhesives were used before synthetic resin adhesives were developed in the modern era, and they are largely classified into organic adhesives and inorganic adhesives. Among the organic adhesives, animal adhesives include shellac, egg white, milk, cheese, and animal glue; plant adhesives include Asian lacquer, natural rubber, pine resin, ulmi cortex, and adhesives extracted from rice and wheat(Yang, 2016). As long periods of time pass, the adhesives are discovered weathered under the ground, or remain in the shape of artifacts, such as craft items and household items. To investigate the types of traditional adhesives, many studies have been conducted to analyze their components. There have been abundant analysis results reported for Asian lacquer, which is known to have excellent durability, because many Asian lacquer samples exist till date.Lu et al. (2013) investigated the components of Asian lacquer and established a marker for identifying lacquer and reported a case study confirming the presence of Asian lacquer in excavated artifacts.Wei et al. (2011) also analyzed a lacquerware artifact discovered in a tomb from the Warring States period in ancient China by using pyrolysis/gas chromatography/mass spectrometry (pyrolysis/GC/MS), and detected 3-pentadecenylcatechol and 3-pentadecylcatechol in the sample and the reference. Then, based on their results that 3-heptylcatechol was detected maximally among the phenol types, they confirmed that the corresponding artifact was lacquerware.Schilling et al. (2016) systematically summarized the results of previous studies related to the analysis of organic materials in Asian lacquer, so that the existence/non-existence and relative composition ratios of various organic composites in Asian lacquer such as drying oils, anacards, anacard oxidation products, proteins, carbohydrates, and resins could be estimated from the pyrolysis/GC/MS analysis results.
In South Korea, there have not been many cases of examining Asian lacquer through instrumental analysis. A few reports include a case of detecting pyrolyzed matter such as phenol, 2-propenylbenzene, 1-tetradecene, and pentadecane by performing pyrolysis/GC/MS analysis on lacquerware samples excavated in Shinchang-dong, Gwangju and Imdang-dong, Gyeongsan(Kim, 2007); a case of detecting components originating from Asian lacquer, such as methylbenzene, 2-hydroxy-4-methylphenol, 3-tetradecene, and pentadecane, by performing pyrolysis/GC/MS analysis on the adhesives used in a great jar of the Proto-Three Kingdoms period(Cho et al., 2010); and a case of confirming an Asian lacquer sample from Nongso Tomb in Sunchang through infrared (IR) and pyrolysis/GC/MS analyses(Naju National Research Institute of Cultural Heritage, 2016). Meanwhile, there have been cases of analysis showing that bone powder was used in the lacquer layer through the detection of calcium and phosphorous. Some examples related to this finding include a case of detecting calcium and phosphorous as main components in the lacquered layer of the dried-lacquer amitabha Buddha statue in Simhyang temple, thereby confirming that bone powder was used(Jeong and Myochin, 2014); a case of detecting calcium and phosphorous in the lacquered layer of a bowl and pot among the lacquerware items excavated at 849-16 Top-dong, Gyeongju, which were determined as artifacts of the Unified Silla period, thereby confirming that bone powder was used (Gyeong-Dam Conservation Laboratory, 2010). In addition, there are many reports confirming the presence of a bone powder layer in the lacquered layer of Najeon-lacquerware items (lacquerware items inlaid with mother-of-pearl) from the Goryeo and Joseon periods(Yi, 1996;Choi et al., 2011). Furthermore, it is mentioned in “On Lacquer Decoration” (Xiushilu, 髹飾錄) that for coating, horn-ash is of the best grade and bone-ash is of medium grade, and it is recorded in “Fundamentals of Lacquer Decoration”(Xiushilu jieshuo, 髹飾錄箋證) that the ash of deer bone is of the best grade, followed by bullhorn(Jeong and Myochin, 2014). It is ascertained from these results that a technique of burning and grinding bones and mixing them to form the coating has been used since ancient times.
1.3. Purpose of research
Following the analysis of the material properties of the glass used in the eyes of the stone standing Maitreya of Daejosa temple(Lee et al., 2018), this study aims to investigate the materials and composition ratio used in the adhesive by analyzing the adhesives fixing the glass eyes. Furthermore, this study estimates the period in which the adhesive was applied to the glass eyes by radiocarbon dating of the adhesive. Thus, the original components of the adhesive are investigated in order to acquire necessary information for the reproduction of adhesive when reinstalling the eyes in the future.
2. EXPERIMENTS
2.1. Materials and reagents
The traditional adhesives used for comparison with the samples were animal glue, dendropanax lacquer, and Asian lacquer. For the animal glue, a small lump of glue(pearl glue, Nakagawa, JPN) was dissolved in water, of which 0.2 mg was added into a pyrolysis cup. For the Asian lacquer, crude lacquer produced in Wonju was dried and ground, and approximately 0.5 mg of the powder was added into a pyrolysis cup. The dendropanax lacquer produced in Wando was dried and ground, and about 0.5 mg of the powder was used. When performing the pyrolysis, 10 μL tetramethylammonium hydroxide solution (TMAH, 25 wt% in water)(Sigma-Aldrich, USA) was used for on-line methylation.
2.2. Adhesive samples
The adhesive samples used were collected when the glass eyes were separated. The outside adhesive exposed to the outside and the inside adhesive existing deep inside the eye holes were classified and analyzed. The collected samples were placed in a mortar and ground to a fine powder until homogeneity was achieved. Then, they were used for IR analysis, pyrolysis/GC/MS analysis, inorganic matter analysis, and radiocarbon dating.
2.3. Analysis methods
2.3.1. Microstructural characteristic analysis
A cross-section of an adhesive sample was fixed with epoxy resin. Then, the cross-section was polished with sandpaper starting from #500 and ending at #2400, and finished with 1 μm and 3 μm abrasives. After polishing, to examine the microstructural characteristics of the cross-section of the adhesive, the surface was examined using a stereoscopic microscope(Stemi 2000C and Axiotech, Carl Zeiss, DEU) and a polarization microscope(Axioplan2, Carl Zeiss, DEU). For microstructural observation and semi-quantitative component analysis, Pt/Pd was coated onto the samples; next, scanning electron microscopy (SEM)(JSM-IT300, Jeol, JPN) with an energy dispersive spectroscopy (EDS) analyzer(X-MAXN, Oxford, GBR) was used. Here, accelerating voltage was 20 kV, probe number was 60, and working distance was set to 10 mm.
2.3.2. IR analysis
For the IR analysis of samples, the attenuated total reflection (ATR) method was used. By using Thermo Fisher Scientific’s Nicolet iS5 model(USA) equipped with a diamond crystal, the measurements were performed by scanning 16 times with 4 cm-1 resolution in the range of 4,000-550 cm-1.
2.3.3. Pyrolysis/GC/MS Analysis
Frontier Lab’s PY-3030D model(JPN) was used for the pyrolyzer, and Agilent’s 7890A GC/5975C MSD model(USA) was used for the GC/MS analysis. A certain amount of sample was added into a pyrolysis cup without special pretreatment; next, it was inserted in a preheated pyrolyzer and pyrolyzed at 500℃ for 0.2 min. Here, when derivatization was required, the pyrolysis was performed after applying 10 μL TMAH. The products of pyrolysis were analyzed on-line by using the GC/MS, and the analysis conditions were as follows. For the analysis column, a DB-1HT column(100% dimethylpolysiloxane, 30 m × 0.25 mm id, 0.10 μm film thickness) was used, and after holding the temperature at 50℃ for 3 min, it was heated to 300℃ at a rate of 10℃/min and maintained at temperature for 5 min, thereby analyzing for a total of 33 min. Helium(0.5 mL/min) was used for the mobile phase gas, and a mass spectrometric detector (MSD) was used for detection. The data acquisition and analysis were performed by using Chemstation software(Agilent Technologies, USA) and NIST MS search program(The National Institute of Standards and Technology, USA).
2.3.4. Inorganic matter analysis
To analyze the inorganic matter contained in the samples, X-ray diffraction (XRD) analysis, X-ray fluorescence (XRF) analysis, and thermogravimetric analysis (TGA) were performed. To identify the minerals composing the adhesives, an XRD analyzer(Empeyrean, PANalytical, NLD) was used. Here, a Cu Kα X-ray source was used with a voltage and current of 40 kV and 40 mA, respectively, the scanning interval was set to 0.02°, and scans were conducted over a range of 10-80°; the diffraction values were recorded using the continuous scanning method. To investigate the composition of the main components of the adhesives, a wavelength-dispersive X-ray fluorescence spectrometer(PW2540, Philips, NLD) was used. The composition ratio of inorganic and organic matter was estimated based on the results of XRF analysis and TGA. In the TGA analysis, a Setsys Evolution(Setaram Inc., FRA) device was used, and according to the measurement conditions, the samples were heated from 0℃ to 1,000℃ at 20℃/min in a N2 gas atmosphere.
2.3.5. Radiocarbon dating
The radiocarbon dating of adhesive samples was done by Beta Analytic Inc.(Florida, USA). The pretreatment of the samples was performed with an acid/alkali/acid method. “Conventional radiocarbon age” was calculated by using the Libby half-life(5568 years) and then compensated for δ13C. The age was rounded off to the nearest decade and shown as radiocarbon years before present(BP age; present = AD 1950). For the conversion of BP age into calendar year, the INTCAL13 database and high-probability density range (HPD) method were used, and the calendar years showing 95.4% and 68.2% probability, respectively, were obtained(Ramsey, 2009;Reimer et al., 2013).
3. RESULTS AND DISCUSSION
3.1. Analysis of microstructural characteristics
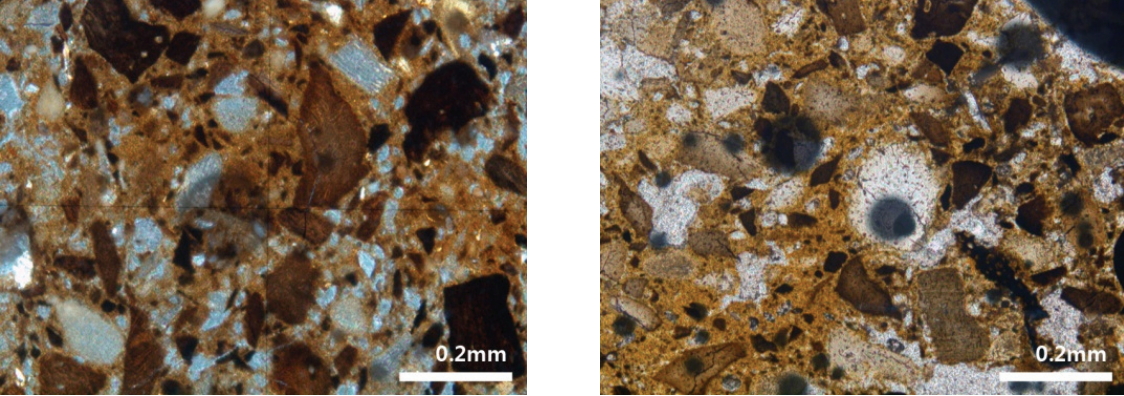
Optical microscope images of the surface of polished adhesives are shown in Figure 3. In the inside and outside adhesives, irregular micro-particles were observed with various colors such as white, gray, and black. The particle size was diverse, up to roughly 400 μm, and black or brown substances were filled in between these particles. In other words, particles of various sizes were embedded within the black and brown matrix. Meanwhile, the matrix showed brown lumps or black belt shapes.
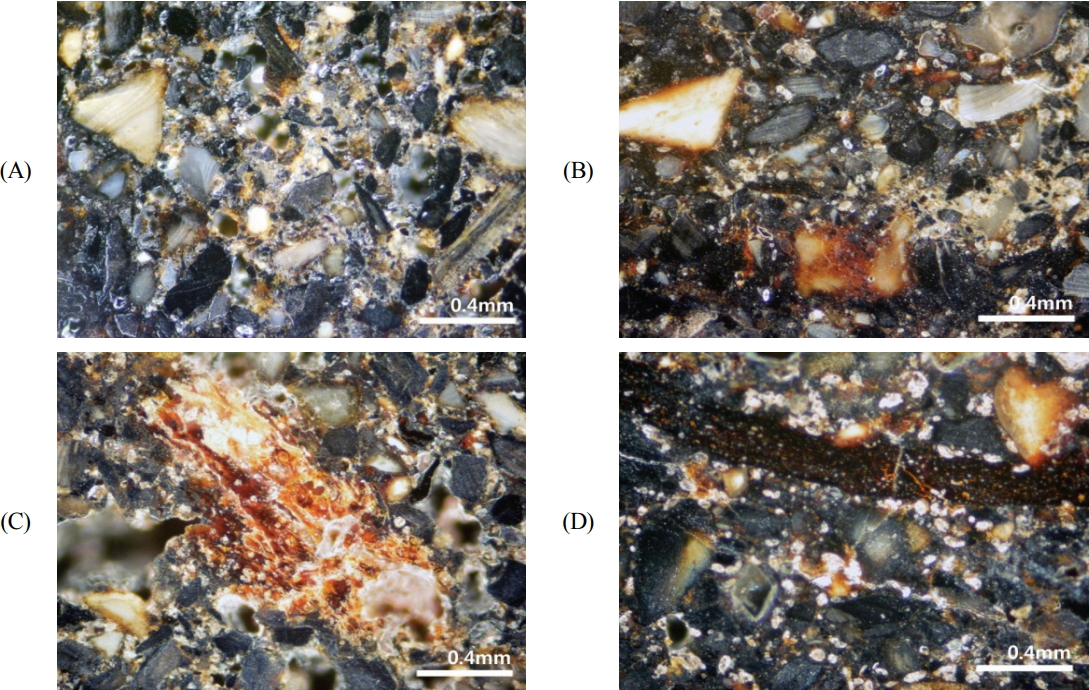
Optical microscopic images of the cross section of the fixing material. (A) Sample from the side of the glass eye and (B)-(D) Sample from the back of the glass eye.
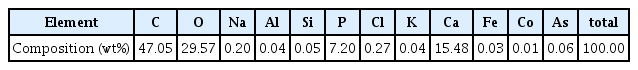
When observed using the polarization microscope after the preparation of a thin slice, the micro-particles did not show the characteristics of minerals, and large porosity was observed (Figure 4). In some, canaliculi or cylindrical structures of the haversian system, a basic unit of bone tissue that comprises the cortical bones of mammals, reptiles, and amphibians, were observed in considerably small amounts(Kang et al., 2014). In addition, the SEM observations confirmed the histological characteristics of the haversian system, a basic unit of bone tissue such as lamellae, lacuna, canaliculi, and harversian canals where blood vessels and nerves pass through. Furthermore, Ca and P were detected as the main components in all the bone micro-particles, which were confirmed as bone tissues. Therefore, it was determined that they were hydroxyapatite, an inorganic material of bone tissue. On the other hand, C was detected in the surrounding matrix, suggesting that the matrix was composed of organic materials(Table 1, Figure 5).
3.2. IR analysis
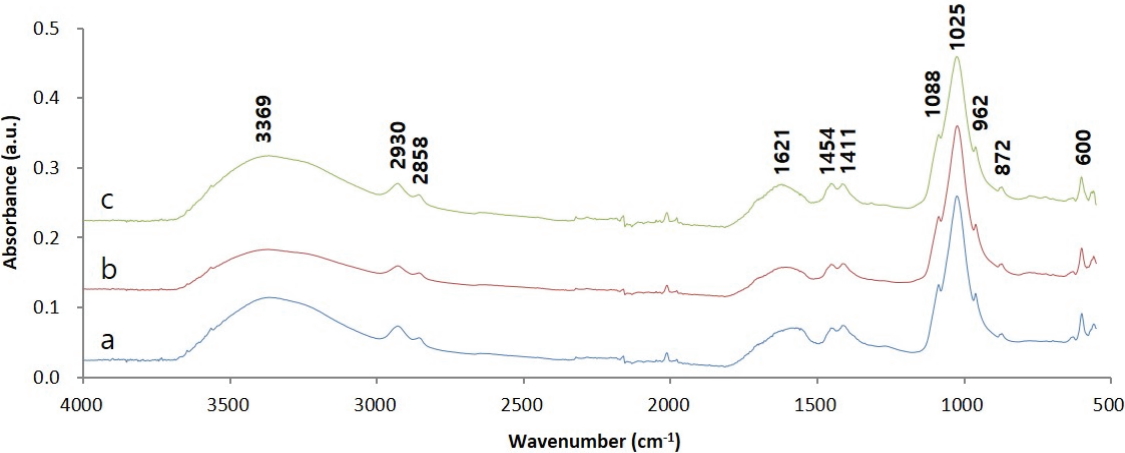
Figure 6 shows the IR spectra of adhesive samples collected from the glass eyes. Considering that the samples from the side and the back of the right glass eye, and the back of the left glass eye all showed the same spectral features, it was determined that the samples were composed of the same materials. First, in the case of an organic material showing a large peak at 3369 cm-1 corresponding to OH stretching, the oxidation seemed to have progressed a lot. The peaks appearing at 2930 and 2858 cm-1 correspond to C-H stretching, indicating this sample contained an organic material. The absorption at around 1025 cm-1 and the absorption at around 1410 cm-1 correspond to C-O stretching(alcohols, ethers, esters, etc.), C-N stretching(amine), C-H bending(-CH3), and C-F stretching in the case of organic materials. However, in the results previously published in the literature, spectral features at wavelengths less than or equal to 1600 cm-1 were consistent with the characteristic spectrum of a material containing phosphates such as bone black, ivory black, and apatite(Lee et al., 2014; Institute of Chemistry University of Tartu, Estonia, 2019). In other words, the peaks at 600, 962, and 1025 cm-1 result from PO43- and those at 872, 1411, and 1454 cm-1 result from CO32-. Therefore, from the IR analysis results, it was ascertained that the adhesives contained inorganic materials containing phosphates along with organic materials. Meanwhile, the IR spectra of dendropanax lacquer, Asian lacquer, and animal glue obtained previously(Park and Lee, 2017) were compared to estimate the type of organic materials; however, it was difficult to find a similarity.
3.3. Pyrolysis/GC/MS analysis
3.3.1. Comparison with traditional adhesives
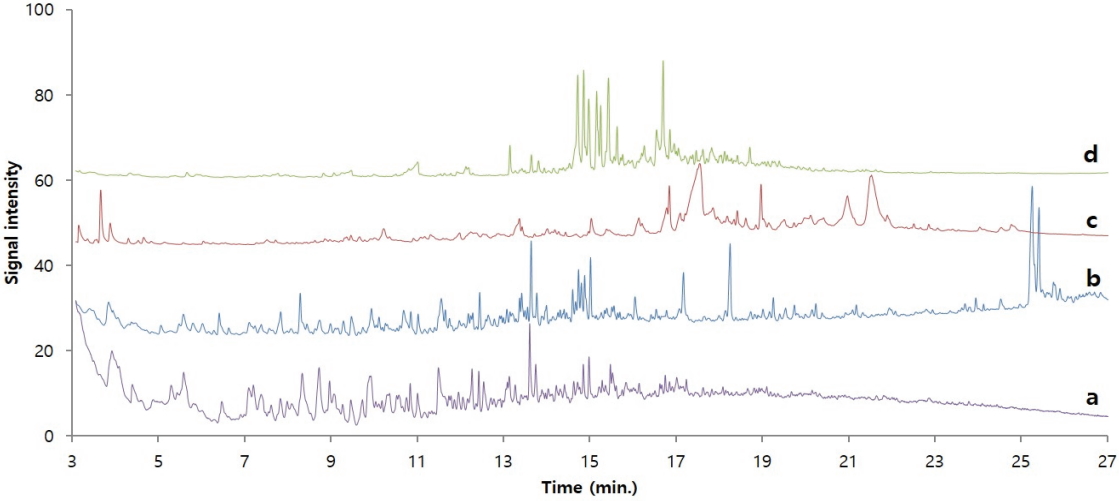
Figure 7 shows the pyrolysis/GC/MS chromatograms of adhesive samples obtained from the glass eyes as well as traditional adhesives(Asian lacquer, animal glue, and dendropanax lacquer). For convenience of comparison, the chromatograms of adhesive samples and animal glue were magnified by ten times and four times, respectively, so that they are of a similar size as the other reference chromatograms. Considering the characteristics of pyrolysis/GC/MS analysis, which shows almost identical chromatograms when the polymer components are the same, it seems there is no substance that shows an identical chromatogram among the samples and three traditional adhesives. However, until approximately 18 min of retention time, the chromatogram of dried Asian lacquer showed a similar pattern as the chromatogram of the adhesive sample. Therefore, there is a strong possibility that the sample contained dried Asian lacquer.
3.3.2. Confirmation of Asian lacquer by on-line derivatization analysis
A polymeric substance, which has been weathered for a long time, contains a lot of organic acids and alcohols as its degradation products. However, organic acids and alcohols are difficult to detect selectively in a hydrophobic column such as a polydimethylsiloxane column because of their strong polarity. To solve this problem, before injecting the sample into the GC, its polarity is decreased by undergoing a derivatization reaction such as methylation and silylation. Here, TMAH and the sample were pyrolyzed together to produce a methylation reaction with the organic acids and alcohols so that the analysis efficiency and sensitivity will be increased. Figure 8 shows the pyrolysis/GC/MS chromatograms of dried Asian lacquer and the adhesive sample obtained through the on-line derivatization process. Here, for the convenience of comparison, the chromatogram of the adhesive sample is shown three times larger than its actual size. First, to investigate the components of the detected peaks, a NIST library search was performed for each peak, and the major components are shown in Table 2 based on the retention time. First, the following compounds were commonly observed: propanoic acid, 2-methoxy-, methyl ester(1), 2,3-dimethoxytoluene(15), tridecane(19), 1,2,4-trimethoxybenzene (21), 1-tetradecene(23), 1-tridecene(27), 1,2-dimethoxy-3-hexylbenzene(32), 1,2-dimethoxy-3-heptylbenzene(35), hexadecanoic acid, methyl ester(37), methyl 7-(2,3-dimethoxyphenyl) heptanoate(38), methyl 8-(2,3-dimethoxyphenyl)octanoate(40), and 1,2-dimethoxy-3-pentadecylbenzene(44). Particularly, 2,3-dimethoxytoluene, 1,2-dimethoxy-3-hexylbenzene, 1,2-dimethoxy-3-heptylbenzene, and 1,2-dimethoxy-3-pentadecylbenzene are derivative components of catechol appearing when urushiol, a main component of Asian lacquer, is pyrolyzed in the presence of TMAH and methylation occurs; they indicate that the adhesive sample obtained from the glass eyes contains Asian lacquer.
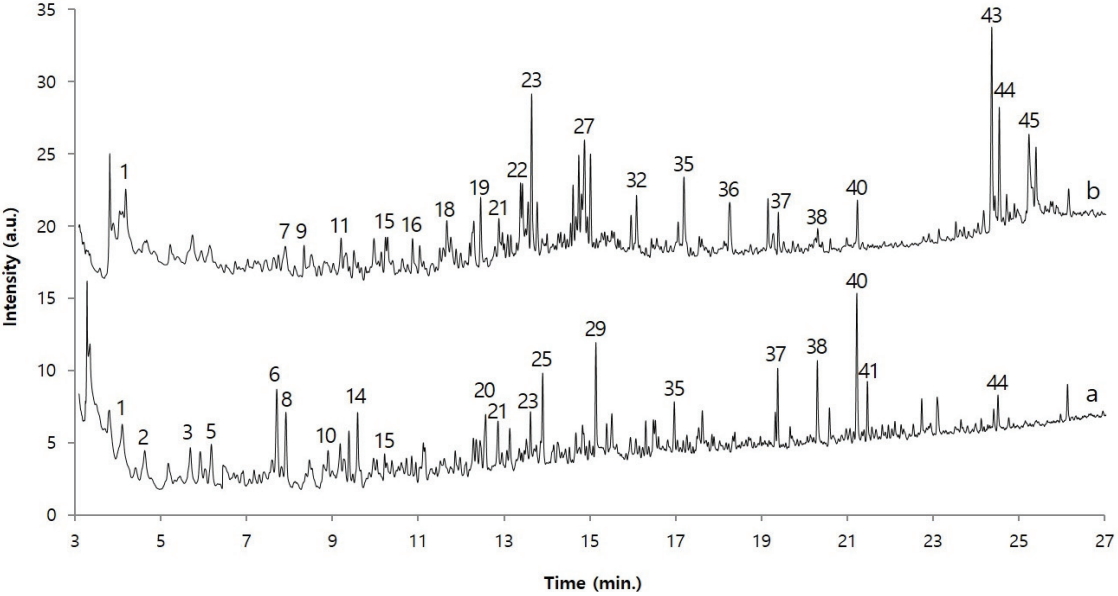
Pyrolysis/GC/MS chromatograms of sample and dried Asian lacquer(on-line methylation with TMAH). (a) Sample (×3) and (b) Dried Asian lacquer.
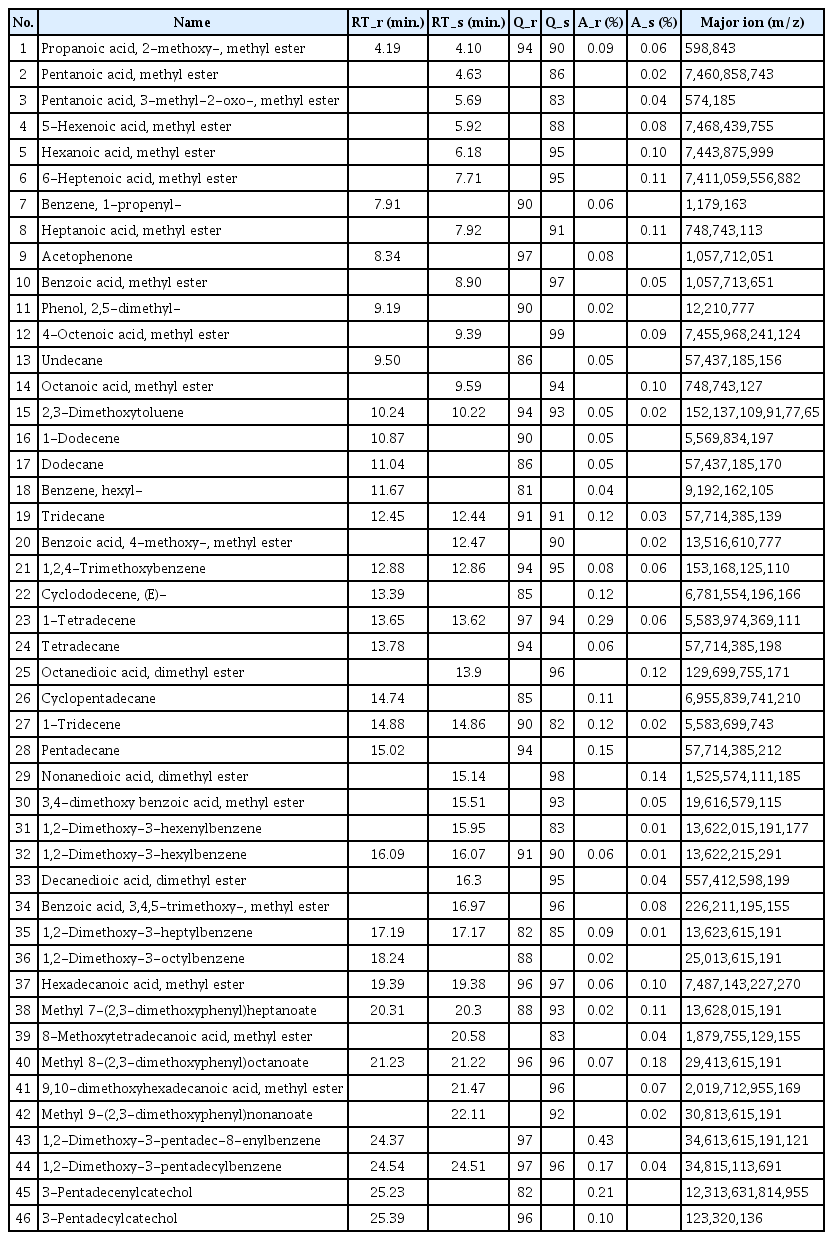
Components of dried Asian lacquer and adhesive sample from the eyes of the stone standing Maitreya of Daejosa temple, Buyeo (Treasure No. 217) analyzed by pyrolysis/GC/MS with on-line methylation
On the other hand, alkanes, alkenes, and catechols were mainly detected in the dried Asian lacquer, and the following acid materials produced by oxidation were detected in large amounts in the adhesive sample obtained from the glass eyes: pentanoic acid methyl ester(2), hexanoic acid methyl ester(5), 6-heptenoic acid, methyl ester(6), heptanoic acid, methyl ester(8), octanoic acid, methyl ester(14), benzoic acid, 4-methoxy-, methyl ester(20), octanedioic acid, dimethyl ester(25), nonanedioic acid, dimethyl ester(29), benzoic acid, 3,4,5-trimethoxy-, methyl ester(34), hexadecanoic acid, methyl ester(37), methyl 7-(2,3-dimethoxyphenyl)heptanoate(38), methyl 8-(2,3-dimethoxyphenyl) octanoate(40), 9,10- dimethoxyhexadecanoic acid, and methyl ester(41). Methyl 7-(2,3-dimethoxyphenyl)heptanoate, methyl 8-(2,3-dimethoxyphenyl) octanoate, and methyl 9-(2,3-dimethoxyphenyl) nonanoate detected in the adhesive sample from the glass eyes are known to be oxidation products of catechol(Schilling et al., 2016). Therefore, it was ascertained that the sample contains oxidized Asian lacquer components. Furthermore, the chromatograms of dried Asian lacquer and the adhesive sample from the glass eyes are not identical, and this seems to be simply because dried Asian lacquer and Asian lacquer that has been placed in a natural state for over hundreds of years have large differences due to weathering.
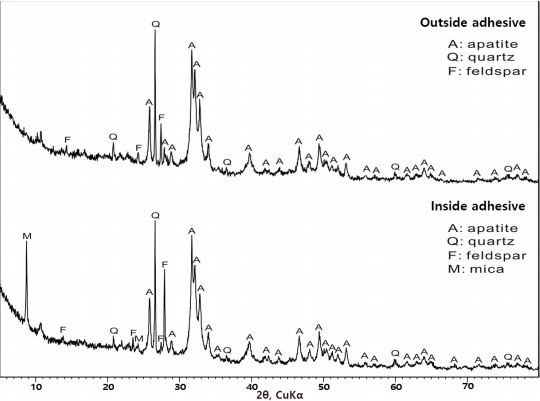
3.4. XRD analysis of adhesive
Apatite [Ca10(PO4)6(OH)2], quartz, and feldspar were identified in the outside adhesive of the glass eyes, and apatite, quartz, feldspar, and mica were identified in the inside adhesive (Figure 9). Based on this, it was determined that the outside adhesive of the glass eyes is mainly composed of apatite and tiny amounts of soil components such as quartz, feldspar, and mica.
3.5. Elemental analysis of adhesive
Elemental analysis of the main components of the adhesive from the glass eyes was performed targeting the inside adhesive only because it was confirmed that the inside and the outside adhesives of the glass eyes contained similar materials through the microstructural characteristics, FT-IR, pyrolysis/GC/MS, and XRD analysis presented above. The results are shown in Table 3. P2O5 and CaO were major components, showing 21.27 wt% and 29.42 wt% content, respectively. In addition, SiO2 and Al2O3 showed 6.22 wt% and 1.17 wt% content, respectively. The remaining components were detected at less than 1 wt%. This is consistent with the results of the above analysis confirming that the inorganic materials of the adhesive contained apatite having Ca and P as main components, and small traces of soil components. In the meantime, the chemical composition of granite is generally known to be 70-77% silica, 11-13% alumina, 3-5% potassium oxide, 3-5% soda, and 2-3% total iron(College of Natural Resources, UC Berkeley, 2019). In the elemental analysis of the adhesive, since the relative proportions of SiO2, Al2O3, K2O, Na2O, and Fe2O3 are similar to those mentioned above, it is assumed that these components came from the granite, the material of the stone Maitreya. Loss on ignition (LOI) is a value calculated from the difference in mass before and after combustion when a sample is burned in a furnace and can be used to estimate the content of organic materials, which are combustible materials. Since the LOI is 39.59%, it seems that the adhesive is composed of roughly 60% inorganic materials and 40% organic materials.
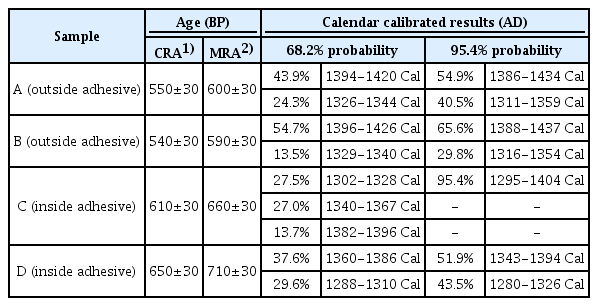
3.6. Radiocarbon dating of adhesive
Table 4 shows the results of radiocarbon dating of the adhesive of the glass eyes(Asian lacquer and bone powder). When the 14C corrected age (95.4% confidence interval) was examined for each sample, the following results were found. For outside adhesive A, age divided into 1386–1434 AD and 1311–1359 AD were calculated. For B, 1388–1437 AD and 1316–1354 AD were calculated. For inside adhesive C, 1295–1404 AD was calculated, and for D, 1343–1394 AD and 1280–1326 AD were calculated. Based on these intervals, it was estimated that the adhesive was applied to the eyes of the stone Maitreya at some time between the late 13th century and early 15th century.
4. CONCLUSION
After analyzing the adhesive used for the glass eyes of the stone standing Maitreya of Daejosa temple, Buyeo, the following conclusions were reached.
1. In the shape of the adhesive, particles of various sizes were embedded in a black and brown matrix. Carbon was observed as the main component of the matrix whereas Ca and P were observed in the particles. Based on the results of chemical component analysis, inorganic materials like P2O5 and CaO account for about 60% of the adhesive, while the LOI accounts for about 40%. Therefore, it was determined that the ratio of inorganic and organic material composition was approximately 60:40. In the XRD analysis, apatite containing Ca and P was identified, indicating that the major inorganic component was apatite.
2. Since the IR spectrum was similar to those of bone black, ivory black, and apatite, it was determined that the major inorganic components originated from bones. Furthermore, in the pyrolysis/GC/MS analysis, since Asian lacquer components and oxidized Asian lacquer components were detected in the adhesive, it seems that the adhesive was based on Asian lacquer and oxidation had progressed with the passage of a long period of time.
3. From the radiocarbon dating results, the carbon date of the adhesive(Asian lacquer and bone black) was determined to be between the late 13th century and early 15th century, indicating that it was used in the stone Maitreya during the late Goryeo period.
Based on the above results, it was ascertained that Asian lacquer and bone black were the main components of the adhesive used for installing the glass eyes on the stone standing Maitreya of Daejosa temple, Buyeo. It seems that the bone black was added as a filler for the purpose of improving the workability and viscosity of the adhesive in order to effectively fix the glass eyes.
Acknowledgements
This study was supported by the National Research Institute of Cultural Heritage (NRICH) as a part of the Cultural Heritage Research & Development program; we, hereby, express our gratitude for this. In addition, we express our gratitude to the Conservation Science Division, NRICH for their cooperation in the inorganic analysis.