 |
 |
- Search
| J. Conserv. Sci > Volume 39(5); 2023 > Article |
|
ABSTRACT
Asian lacquer is a material that has been used in Asia from ancient times to coat the surface of objects. It is a durable material, but is known to be weak to ultraviolet light (UV). Here, to understand the deterioration characteristics of Asian lacquer due to UV, the changes were analyzed using ultraviolet photometry, IR, and pyrolysis/GC/MS (pyrolysis/GC/MS), after exposing the lacquer coating film to UV (λ = 340 nm). In the ultraviolet photometry, the surface exposed to UV showed fluorescence. In IR analysis, the powder sample showed little change after exposure to UV, but on the surface, C = O groups increased, while C―H groups decreased significantly. Analysis with pyrolysis/GC/MS revealed that the composition of organic acid increased significantly. Carboxylic acids having 9 or less carbon atoms and dicarboxylic acids were significantly increased compared to carboxylic acid having 16 carbon atoms. In addition, urushiol with the oxidized side chain was also increased. This shows that the penetration depth of UV is the limiting factor for deterioration, and that the deterioration of lacquer due to UV proceeds with the destruction of carbon–carbon single and double bonds and the subsequent formation of carboxylic acid.
Asian lacquer, a sap from lacquer trees, forms a strong and hard film while drying, and has effects such as waterproofing, antiseptic, and insect repellent, and is a material with good chemical resistance. It has been used as a natural paint or adhesive in East Asia, particularly Korea, China, and Japan, since ancient times, and has been made into living appliances and crafts, such as wood, paper, textile, ceramics, and metal. Lacquered objects have been buried in the ground for thousands of years, and passed down to us today. It is known that even such durable lacquer deteriorates, especially when exposed to UV, causing flaking, loss in gloss, dulling, and cracking. Therefore, there have been various studies to observe these changes in detail, and elucidate the deterioration process. Studies describing the phenomenon that occurs when a lacquer sample is irradiated with UV are as follows. Araki observed the changes in color of lacquer sample exposed to 365 nm UV light (Araki and Sato, 1978), while Kamiya measured the changes in gloss (Kamiya and Kato, 2006). Nakagoshi and Ogawa observed the degree of deterioration by changing the wavelengths of light in the UV and visible regions (Nakagoshi and Yoshizumi, 2011; Ogawa et al., 2001). Studies observing functional groups and surface changes of lacquer film by exposure to sunlight and fluorescent lamps (Ogawa et al., 1993; Ogawa et al., 1998), and one observing the formation of a cavity by immersing a lacquer film in water after UV exposure (Shimadzu and Kawanobe, 2003), were also reported. Kim observed the change in the cross-section of a lacquer film having various types of layers after UV irradiation (Kim, 2007). Yamashita described the deterioration of lacquerware artifacts in detail, and presented the stages of deterioration by light (Yamashita and Rivers, 2011). Studies to elucidate a deterioration mechanism have also been attempted. There is a report that almost all UV radiation was absorbed by the lacquer film, leading to the decomposition of the urushiol polymer, thereby reducing the weight of the film (Toyoshima, 1996). Film thickness decreased with cleansing by water as the deteriorated urushiol and polysaccharide were washed away, because after being irradiated with UV, urushiol is decomposed into a low molecular weight compound (Oyabu et al., 1998). After exposing the lacquer film (black lacquer with iron) to UV, Kamiya observed the change in the surface of the lacquer film by IR, pyrolysis/GC/MS, and XPS, and interpreted it in the context of chemical bond formation and breakage (Kamiya et al., 2006). In addition, a mechanism whereby oxidative cleavage occurs in the urushiol polymer in the process of deterioration has been suggested from the detected compounds by the online monitoring of volatile organic compounds generated during UV irradiation for 30 h (Kamiya et al., 2011). Meanwhile, Liu modeled the ageing behavior by measuring the viscoelastic response of UV-degraded lacquer film (Liu et al., 2011). Hong observed that when it was irradiated with 313 nm light, the mass of a lacquer film decreased and a large hole was formed on the surface, and reported that it could be reduced by adding hindered amine light stabilizer (Hong et al., 2000). The gloss and color of lacquer film being stabilized by bromination of its surface has also been reported (Ogawa et al., 2002). However, there has been no study explaining the difference in deterioration across the layers by comparing the composition changes of the surface and the entire film of lacquer film caused by UV irradiation.
The purpose of this study is to understand the deterioration phenomenon of Asian lacquer. It is known that Asian lacquer, which has strong durability, is especially weak to UV light. Here, after exposing the lacquer film to UV light, it was analyzed with IR and pyrolysis/GC/MS by dividing the surface and the entire film to get a compositional change. According to their origin, the constituents of lacquer were classified into 6 categories to interpret the complicated pyrolysis/GC/MS data in concise manner. The changes in occupation of such categories were examined in detail to get a glimpse of the deterioration process of UV-irradiated lacquer film.
Asian lacquer was collected from lacquer trees grown in Wonju, Gangwon-do, South Korea, and used after removing impurities. A thin layer of liquid lacquer sap was applied on a glass plate, and dried at 21.1°C and 80% RH for 14 days (d) to prepare a dried film. The glass plate of 7 cm × 14 cm size was washed thoroughly with distilled water, and dried.
The accelerated ageing of the lacquer film by UV was in accordance with ASTM D4303-10. The ageing environment was implemented using ATLAS Ci4000 Xenon Weather-o-meter and the black panel temperature of (63±2)°C, RH of (55±5)%, and irradiance of (0.35±2) W/m2 at 340 nm were applied. After dividing the lacquer film on the glass plate into 5 zones, all except 1 zone were wrapped with aluminum foil, and UV ageing was then started. After 6 d, zone 1 was irradiated with UV for 6 d. Then, zones 1 and 2 were exposed to UV, and further aged for 6 d. After that, zone 1 was irradiated with UV for 12 d, and zone 2 for 6 d. In this way, samples of the dried lacquer film exposed to UV for (0, 6, 12, 18, and 24) d, respectively, were obtained. A sample stored at room temperature (RT) with no light was also prepared for comparison.
The ultraviolet photograph of the sample was taken using a PH135 UV spotlight (Labino AB, SWE) as a light source. This light source is characterized by power = 35 watt, wavelength = 365 nm, intensity = 45,000 μW/cm2. The light source was irradiated on the sample while a photograph was taken using a digital camera.
IR analysis was conducted by attenuated total reflection (ATR) method equipped with Ge crystal using Hyperion 3000 (Bruker, DEU). A powder and a surface sample were applied. Powder sample was prepared as fine as possible by putting and crushing the lacquer film in an agate bowl to ensure homogeneity. Surface sample was prepared as described in the previous section. Samples were scanned 64 times with a resolution of 4 cm−1 in the range (4,000―600) cm−1.
Thermally assisted hydrolysis and methylation (THM)-pyrolysis/GC/MS analyses were performed on a Frontier PY-2020D micro-furnace pyrolyzer interfaced to an Agilent 7890A GC/5975C inert MSD. The surface sample for pyrolysis/GC/MS was collected by lightly scraping the surface of the sample using a surgical knife. About (25―60) μg of the sample was placed into 50 μL stainless steel Eco-cups with 3 μL of tetramethylammonium hydroxide (TMAH) solution (25% in methanol), and pyrolyzed at 550°C for 20 s. The analysis conditions for GC/MS were as follows. A J&W DB-5 ms capillary column (100% dimethylpolysiloxane, 20 m × 0.18 mm × 0.18 μm) attached to a Frontier Vent-Free adaptor was used with the helium flow set to 1 mL/min. The split injector was set to 320°C with a split ratio of 20:1. The oven of the GC was held at 35°C for 1 min, then ramped to 110°C at 60°C/min, to 240°C at 14°C/min, and to 290°C at 5°C/min, followed by a 3 min isothermal hold (a total time of 24.5 min).
AMDIS distributed by NIST was used to retrieve compounds from the pyrolysis/GC/MS chromatogram. The results were interpreted with a RAdICAL workbook developed by the Getty Conservation Institute, USA (Schilling et al., 2016). This program based on Excel classifies the compounds of pyrolysis/GC/MS chromatogram into Anacards, Fatty acids and oils, Resins, Proteins, Carbohydrates, and Miscellaneous materials, according to their marker compounds. Compounds like catechols, acid catechols, phenols, and hydrocarbons originating from the saps of lacquer-producing trees in the Anacardiaceae family (Anacard sap) fall into the Anacards class. Compounds like monocarboxylic fatty acids, dicarboxylic fatty acids, and glycerol fall into Fatty acids and oils. Compounds from Pinaceae, cedar oil, pitch, camphor, gum benzoin, and so on, fall into Resins. Markers related to proteinaceous materials, like blood, egg, and animal glue, fall into Proteins. Starch, tofu, paper, and carbohydrate originating from the Anacard sap are classified as Carbohydrates. This program also gives the composition of each category in the sample from the peak area of the chromatogram. In addition, the peak areas of individual compound constituting the categories help to elucidate the composition of the sample in a semi-quantitative manner.
Figures 1a & b show the photographs of the lacquer film before and after exposure to UV. The exposure time is (24, 18, 12, and 6) d, and no exposure from the far right, respectively. Compared to the sample before exposure (Figure 1a, it was observed that when placed in the weather-o-meter for 24 d, the color of the lacquer film turned brighter and more transparent, regardless of UV exposure (Figure 1b). It seems that in the experimental condition of 63°C, the molecular structure of lacquer is changed by heat. However, the picture obtained under an ultraviolet light source (peak wavelength = 365 nm) is different (Figure 1c). Fluorescence was observed in the areas exposed to UV for (24, 18, 12, and 6) d, different from the unexposed one on the far left. It seems that lacquer film was affected by UV to change the molecular structure. On the other hand, the back side of the sample before and after UV exposure showed no difference under UV photometry. UV appears to show a confined penetration in the direction of the lacquer film depth. Fluorescence was observed in all samples exposed for (6 to 24) d. So, IR and pyrolysis/GC/MS analysis was performed just on samples exposed on d (0 and 6), together with a sample stored at RT for comparison.
Figure 2 shows IR spectra obtained by the ATR method shown for the powder samples of the lacquer film. Except for a slight increase in absorption near 1,718 cm−1 corresponding to C = O stretching, there is no significant change in the spectra before and after UV exposure. In line with the above UV photo results, UV shows little effect on the film as a whole.
Figure 3 shows the IR spectra of the lacquer film surface measured by the ATR method. The ATR method reflects the properties of the film surface, because the measurement depth is of the order of several μm. IR spectra of the sample stored at RT, and that in the weather-o-meter without UV irradiation, did not change significantly, except for a slight decrease in absorption at 991 cm−1. This 991 cm−1 corresponds to CH bending of the mono-substituted alkene, and it seems that the reaction occurred at the double bond of the side chain when the lacquer film was placed at the temperature of 63°C. When exposed to UV for 6 d, a very large spectral change was observed, different from the powder sample. The absorption at (2,925 and 2,854) cm−1 corresponding to the CH single bond decreased sharply, whereas the absorption corresponding to C = O near 1,724 cm−1 increased significantly. The O―H stretching around 3,400 cm−1 also changed. Absorption at 3,268 cm−1, which is presumed to be carboxylic acid O―H, appeared with UV exposure. In addition, the absorption pattern in the region of below 1,500 cm−1 was also changed. The absorption at 1,456 cm−1 corresponding to CH2 bending decreased with UV exposure. From the decrease in absorption at (2,925, 2,854, and 1,456) cm−1, it is thought that the decomposition of the urushiol side chain was occurring. The absorption of 1,184 cm−1 associated with the acyl C―O stretching of the ester also increased with UV exposure. On the other hand, absorption at (991 and 723) cm−1 corresponding to C = C bending (= C―H out-of-plane bend) decreased with UV exposure. In particular, decrease in absorption at 723 cm−1 corresponding to di-substituted alkene or di-substituted aromatic indicates that decomposition was occurring in both the side chain and the benzene ring of urushiol. From this, it can be estimated that the C―C and C = C bond of the benzene ring and side chain of urushiol on the surface of the lacquer film were decomposed by UV to produce carboxylic acid.
pyrolysis/GC/MS analysis was performed on a surface sample of the lacquer film from the above finding that UV affects only the surface of the film. Figure 4 and Table 1 show the pyrolysis/GC/MS chromatograms of the surface samples of lacquer film, and the compounds corresponding to each peak, respectively. Here, compounds were searched using the AMDIS program distributed by NIST.
pyrolysis/GC/MS analysis results were further analyzed using the RAdICAL workbook described in section 3.6. The compounds in the pyrolysis/GC/MS chromatogram were classified into Anacards, Fatty acids and oils, Resins, Proteins, Carbohydrates, and Miscellaneous materials by each marker compound. The peak areas of individual compounds comprising the categories were put together to give the composition of each category in the sample in a semi-quantitative manner. When these compounds were classified and the change in the composition ratio by category was examined, changes in the components that were difficult to grasp by just observing the chromatogram pattern could be seen (Table 2).
The Fatty acids and oils slightly increased in the samples in the chamber without UV radiation in comparison with the one stored at RT. When exposed to UV light for 6 d, the change was remarkably large with the composition of Anacards greatly reduced to 19.4%, while that of the Fatty acids and oils increased significantly to 61.5%. It seems that catechols, acid catechols, and phenols were decomposed and oxidized by UV to produce mono- and di-carboxylic fatty acids. Meanwhile, the materials classified as Other increased rapidly to 13.4%, including 1,2,3-trimethoxybenzene, 1,2,4-trimethoxybenzene, 1,2,3,4-tetramethoxybenzene, methyl methoxyacetate, and methyl 2,3-dimethoxybenzoate as main compounds. These are presumed to be the substances produced by over-oxidation or decomposition of catechol by UV. From this, it can be said that UV greatly accelerates the oxidation of the surface of the lacquer film.
A detailed examination of fatty acids shows that nonanedioic acid was generated in a sample placed in weather-o-meter in comparison with that stored at RT (Figure 5). This means that when heat is applied to Asian lacquer, this compound known as a characteristic component of drying oil (Wang et al., 2015) can be produced, even though there is no addition of drying oil. In the sample exposed to UV, various carboxylic acids were produced; in the meanwhile, palmitic acid was greatly reduced. It can be said that the decomposition of lacquer polymers including palmitic acid and subsequent oxidation by UV formed low molecular weight carboxylic acids.
Figure 6 shows the changes in relative abundance of the catechol sub-class, a major component of Asian lacquer. Firstly, a large amount of acid catechol is observed in a sample placed in the weather-o-meter without UV, which is very different from the sample stored at RT. Heating in the chamber seemed to promote the oxidation of catechol. Urushiol has a side chain of 15 carbons in catechol with 1 to 3 double bonds. Some of them undergo auto-oxidation, and cross-linking occurs in the polymer chain. It is presumed that in the presence of heat, peroxyradicals are decomposed and converted to oxyradicals, and then through oxidation, converted to acids (Lu and Miyakoshi, 2015). As a result, it appears that in samples exposed to heat, acid catechol is increased. Under UV irradiation, this phenomenon continues, and the portion of C8 acid catechol becomes larger than that of C15 catechol, to become the dominant catechol. Catechol seemed to be oxidized and continuously converted to acid catechol. This is in accordance with the report that C7 and C8 acid catechols were detected in large amounts, in comparison with nearly no detection of C15 catechol in excavated lacquerware that had undergone severe weathering in the ground (Park et al., 2018a, 2018b). Li et al. reported that catechol adsorbed on silica is converted to semiquinone, and eventually decomposes to carbon dioxide and water with the breakage of benzene ring under light irradiation conditions in the presence or absence of oxygen (Li et al., 2016). So, it is estimated that as the lacquer film is exposed to UV, decomposition of catechol or acid catechol proceeds to form small-chain carboxylic and dicarboxylic acids, resulting in the large increase of Fatty acids and oils in the sample with UV irradiation of 6 d, as described above.
The compositional changes of the lacquer film with irradiation of UV were analyzed by IR and pyrolysis/GC/MS. The IR analysis found that just the surface, not the whole of the lacquer film, was changed by UV, and that mainly hydrocarbon and benzene rings were broken and oxidation reaction occurred. When the pyrolysis/GC/MS analysis results were interpreted by classifying them into Anacards and Fatty acids and oils, the proportion of Anacards decreased, while that of Fatty acids and oils significantly increased, due to the exposure to UV. Short-chain carboxylic and dicarboxylic acids increased, with a decrease of long-chain carboxylic acids. Catechols decreased, while the amount of oxidized acid catechols increased. From these observations, it is presumed that the deterioration of Asian lacquer due to UV proceeds through the decomposition of the benzene rings and polymer chains by UV, and subsequent reaction with oxygen to produce fatty acids. So, to slow down the ageing of Asian lacquerware, it is needed to store it away from UV rays such as sunlight.
ACKNOWLEDGEMENT
This work was supported by the Getty Conservation Institute under the 2016/2017 Conservation Guest Scholar Program. The support by the National Research Institute of Cultural Heritage, Republic of Korea, is gratefully acknowledged.
Figure 1
Photographs of dried lacquer film on glass plate. (a) Before UV aging experiment (The exposure time is (24, 18, 12, and 6) day, and no exposure from the far right), (b) After UV aging, (c) After UV aging taken with UV photometry (wavelength = 365 nm).

Figure 2
IR spectra of bulk powder sample of the dried lacquer film according to aging time under UV irradiation. (a) Stored at RT without light, (b) In the weather-o-meter without UV irradiation, (c) After UV irradiation of 6 days. The film was powdered for analysis.
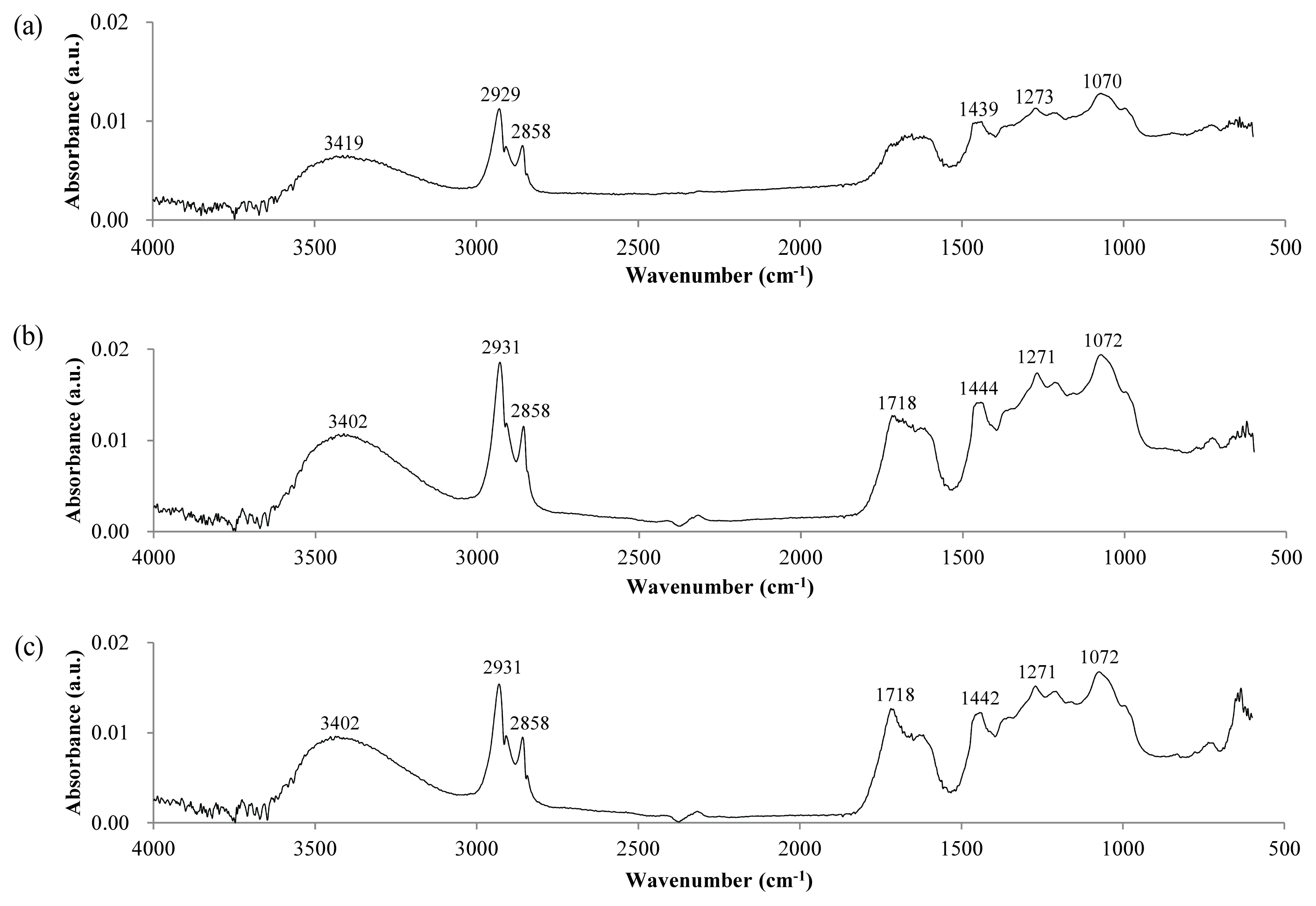
Figure 3
IR spectra of the surface of dried lacquer film after UV irradiation obtained by the ATR method. (a) Stored at RT without light, (b) In the weather-o-meter without UV irradiation, (c) After UV irradiation of 6 d.
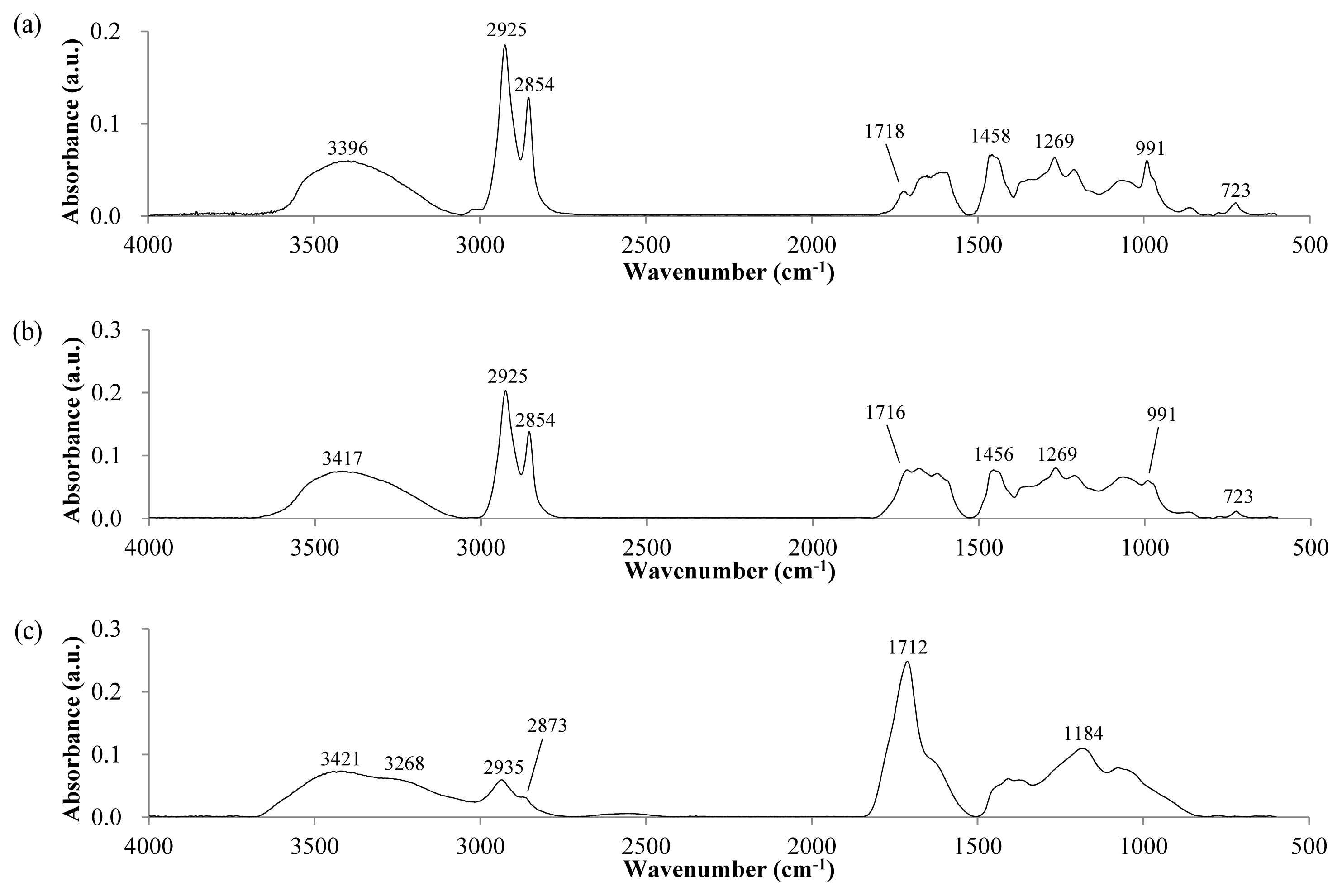
Figure 4
Pyrolysis/GC/MS chromatograms of the dried lacquer film according to aging time under UV irradiation. (a) Stored at RT without light, (b) in the weather-o-meter without UV irradiation, (c) after UV irradiation of 6 d. The surface of the film was scratched to get a sample.
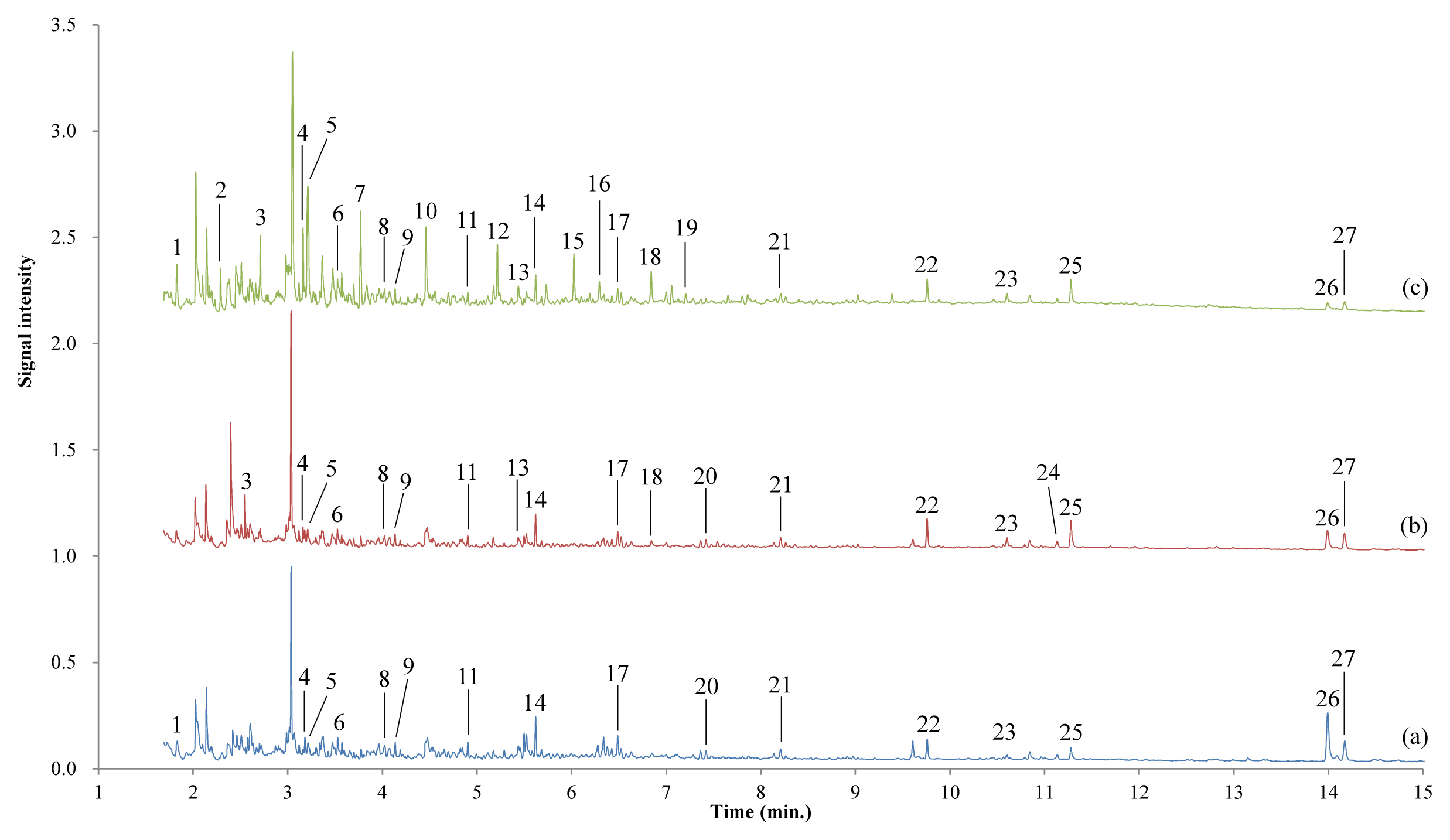
Figure 5
Changes in fatty acids and oils of surface sample of the dried lacquer film with UV irradiation. (a) Stored at RT without light, (b) In the weather-o-meter without UV irradiation, (c) After UV irradiation of 6 d. C4 stands for mono-or dicarboxylic acid with 4 carbons, C5-1 stands for carboxylic acid with 5 carbons with a double bond, and so forth.
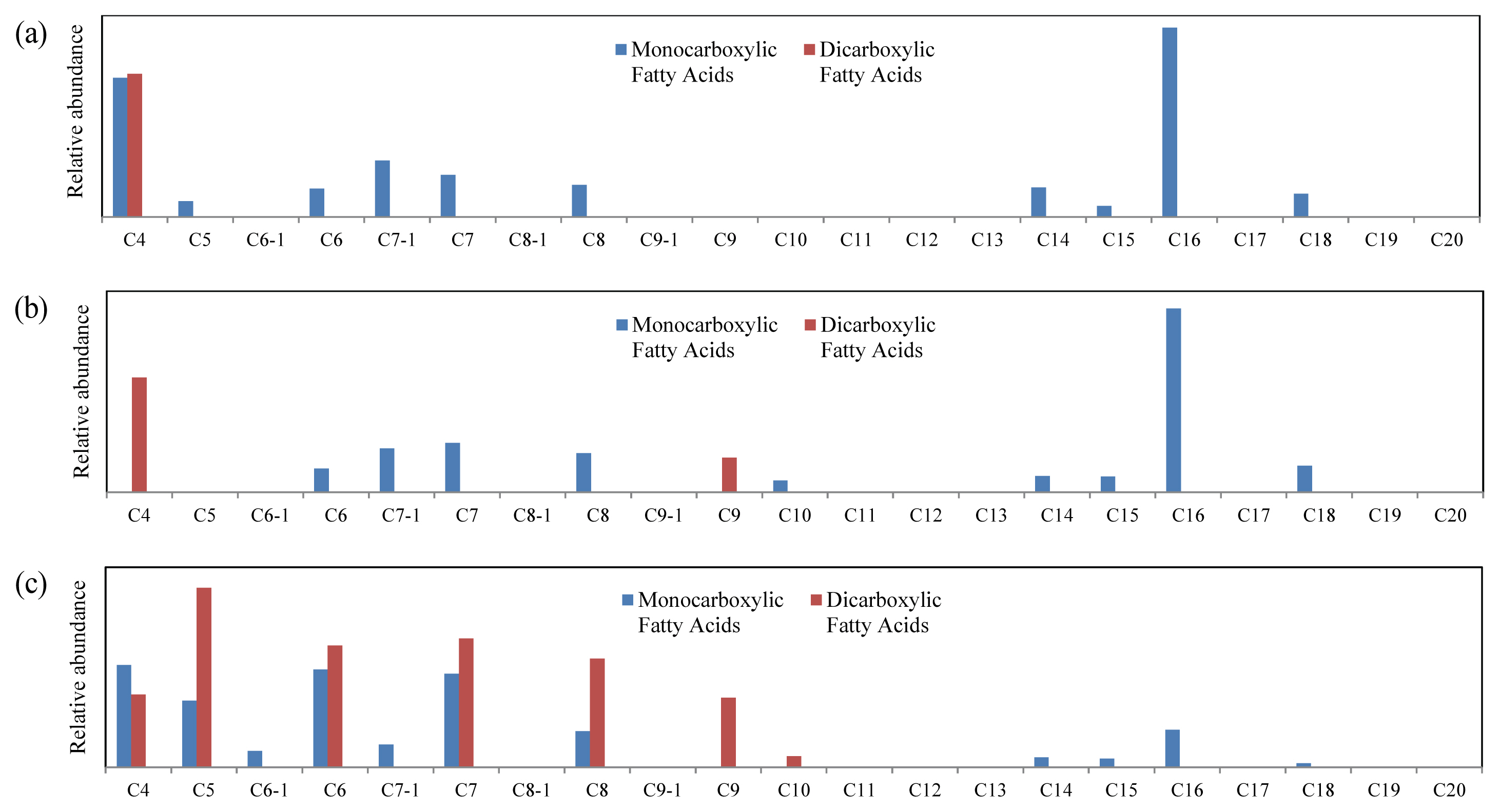
Figure 6
Changes in anacards constituents of surface sample of dried lacquer film with thermal aging and UV irradiation. (a) Stored at RT without light, (b) In the weather-o-meter without UV irradiation, (c) After UV irradiation of 6 d. C15-1 catechol denotes catechol with 15-carbon chain on its benzene ring with a double bond, C8 acid catechol for catechol with 8-carbon carboxylic acid on its benzene ring, and so forth.

Table 1
The compounds of the labeled peaks in the pyrolysis/GC/MS chromatograms of dried lacquer film before and after UV light ageing
| No. | Retention time (min) | Namea | Major ions (m/z) |
|---|---|---|---|
| 1 | 1.83 | Butanoic acid, methyl ester | 43, 74, 71, 59, 87 |
| 2 | 2.3 | Pentanoic acid, methyl ester | 74, 57, 85, 87, 101 |
| 3 | 2.71 | Hexanoic acid, methyl ester | 74, 87, 43, 99, 59 |
| 4 | 3.16 | Heptanoic acid, methyl ester | 74, 87, 43, 113, 144 |
| 5 | 3.21 | Butanedioic acid, dimethyl ester | 115, 55, 59, 114, 87 |
| 6 | 3.54 | Phenol, 2-methoxy- | 109, 124, 81, 53, 63 |
| 7 | 3.77 | Pentanedioic acid, dimethyl ester | 59, 100, 129, 55, 128 |
| 8 | 4.03 | 2,3-Dimethoxytoluene | 152, 137, 109, 91, 77 |
| 9 | 4.14 | 1-Dodecene | 41, 69, 97, 111, 168 |
| 10 | 4.46 | Hexanedioic acid, dimethyl ester | 59, 114, 101, 111, 143 |
| 11 | 4.9 | Tridecane | 57, 71, 85, 99, 184 |
| 12 | 5.22 | Heptanedioic acid, dimethyl ester | 115, 74, 55, 125, 157 |
| 13 | 5.44 | 1,2,4-Trimethoxybenzene | 153, 168, 125, 110, 69 |
| 14 | 5.62 | 1-Tetradecene | 43, 69, 97, 125, 196 |
| 15 | 6.03 | Octanedioic acid, dimethyl ester | 129, 138, 74, 97, 171 |
| 16 | 6.3 | Benzoic acid, 2,3-dimethoxy-, methyl ester | 163, 164, 196, 107, 122 |
| 17 | 6.49 | Pentadecane | 57, 71, 85, 99, 212 |
| 18 | 6.84 | Nonanedioic acid, dimethyl ester | 152, 55, 111, 143, 185 |
| 19 | 7.21 | 3,4-dimethoxy benzoic acid, methyl ester | 165, 196, 79, 121, 137 |
| 20 | 7.42 | 1,2-Dimethoxy-3-hexylbenzene | 136, 222, 152, 91, 77 |
| 21 | 8.21 | 1,2-Dimethoxy-3-heptylbenzene | 136, 236, 152, 151, 91 |
| 22 | 9.76 | Hexadecanoic acid, methyl ester | 74, 87, 143, 270, 227 |
| 23 | 10.6 | Methyl 7-(2,3-dimethoxyphenyl)heptanoate | 136, 280, 151, 91, 121 |
| 24 | 11.14 | Octadecanoic acid, methyl ester | 74, 87, 127, 115, 158 |
| 25 | 11.28 | Methyl 8-(2,3-dimethoxyphenyl)octanoate | 136, 294, 151, 91, 121 |
| 26 | 13.99 | 1,2-Dimethoxy-3-pentadec-8-enylbenzene | 346, 136, 151, 152, 91 |
| 27 | 14.17 | 1,2-Dimethoxy-3-pentadecylbenzene | 348, 151, 136, 152, 91 |
Table 2
Composition of dried lacquer film before and after UV light ageing (%)
REFERENCES
Araki, T. and Sato, H., 1978, Relationships between exhibition lighting and discoloration of lacquered wares. Kobunkazainokagaku, 23, 1–24.
Hong, J.H., Park, M.-Y., Kim, H.-K. and Choi, J.-O., 2000, UV-degradation chemistry of oriental lacquer coating containing hindered amine light stabilizer. Bulletin of the Korean Chemical Society, 21(1), 61–64.
Kamiya, Y. and Kato, H., 2006, Assessment of the surface of lacquer films deteriorated by ultraviolet irradiation. Bunkazaihozonshufukugakkaishi: Kobunkazainokagaku, 51, 51–58.
Kamiya, Y., Lu, R., Kumamoto, T., Honda, T. and Miyakoshi, T., 2006, Deterioration of surface structure of lacquer films due to ultraviolet irradiation. Surface and Interface Analysis, 38(9), 1311–1315.

Kamiya, Y., Takeda, S., Watanabe, C. and Miyakoshi, T., 2011, Characterization of volatile products from raw lacquer film during ultraviolet irradiation using on-line micro ultraviolet pyrolysis-GC/MS. Bunsekikagaku, 60(3), 269–74.

Kim, S.C, 2007, Analysis and conservation of lacquer wares from archaeological sites in Korea. Ph.D. Dissertation, Chungbuk National University, Cheongju, 11–108.
Li, H., Guo, H., Pan, B., Liao, S., Zhang, D., Yang, X., Min, C. and Xing, B., 2016, Catechol degradation on hematite/silica–gas interface as affected by gas composition and the formation of environmentally persistent free radicals. Scientific Reports, 6, 244941–9.



Liu, X., Elmahdy, A.E., Wildman, R.D., Ashcroft, I.A. and Ruiz, P.D., 2011, Experimental investigation and material modelling of fresh and UV aged Japanese lacquer (Urushi). Progress in Organic Coatings, 70(4), 160–69.

Lu, R. and Miyakoshi, T., 2015, Lacquer chemistry and applications, Elsevier, Amsterdam, 101–102.
Nakagoshi, K. and Yoshizumi, K., 2011, Degradation of Japanese lacquer under wavelength sensitivity of light radiation. Materials Sciences and Applications, 2(10), 1507–1515.

Ogawa, T., Arai, K. and Osawa, S., 1998, Light stability of oriental lacquer films irradiated by a fluorescent lamp. Journal of Environmental Polymer Degradation, 6(1), 59–65.
Ogawa, T., Jinnai, H. and Osawa, S., 2001, Wavelength dependence on light deterioration of oriental lacquer film. Kobunshironbunshu, 58(9), 442–47.

Ogawa, T., Jinnai, H. and Osawa, S., 2002, Stabilization in appearance of oriental lacquer film by bromination. Kobunshironbunshu, 59(3), 127–132.

Ogawa, T., Yabu, T. and Sakamoto, M., 1993, Surface analyses of urushi film deteriorated by outdoor exposure. Kobunkazainokagaku, 38, 37–44.
Oyabu, H., Asami, T. and Ogawa, T., 1998, Deterioration process of Urushi films by accelerated weathering test. Materiaruraifu, 10(1), 43–51.
Park, J., Cho, H. and Lee, J., 2018, Organic material analysis of a lacquered wooden sheath of long sword with ring pommel excavated in Imdang ancient tomb. Journal of Conservation Science, 34(5), 369–377.

Park, J., Schilling, M.R., Khanjian, H. and Lee, J., 2018, Stratigraphic examination of a Korean lacquered wooden coffin sample pyrolysis/GC/MS. Chromatographia, 81, 1685–1694.

Schilling, M.R., Heginbotham, A., van Keulen, H. and Szelewski, M., 2016, Beyond the basics – A systematic approach for comprehensive analysis of organic materials in Asian lacquers. Studies in Conservation, 61(sup3), 3–27.

Shimadzu, Y. and Kawanobe, W., 2003, Deterioration phenomena of urushi films – combined effects of ultraviolet radiation and water. Shikizaikyokaishi, 76(10), 385–390.

Toyoshima, K., 1996, Study of deterioration of Urushi film with ultraviolet radiation. Materiaruraifu, 8(1), 28–35.
Wang, N., He, L., Zhao, X. and Simon, S., 2015, Comparative analysis of eastern and western drying oil binding media used in polychromic artworks by pyrolysis-gas chromatography/ mass spectrometry under the influence of pigments. Microchemical Journal, 123, 201–210.

Yamashita, Y. and Rivers, S., 2011, Light-induced deterioration of urushi, maki-e and nashiji decoration. In: Rivers S., Faulkner R., Pretzel B., editors. East Asian Lacquer: Material Culture, Science and Conservation, London, Archetype Publications, 208–216.
- TOOLS






